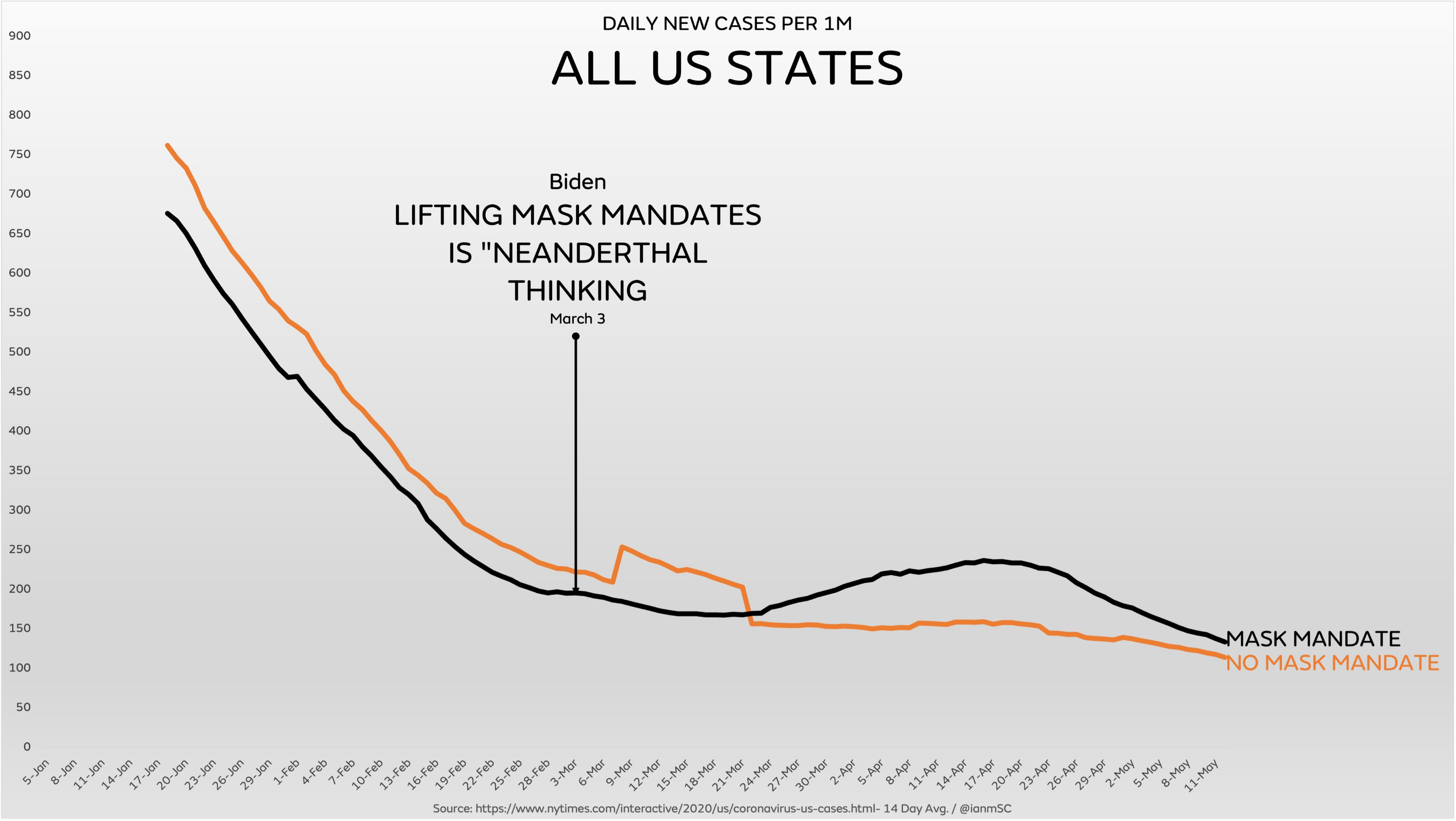
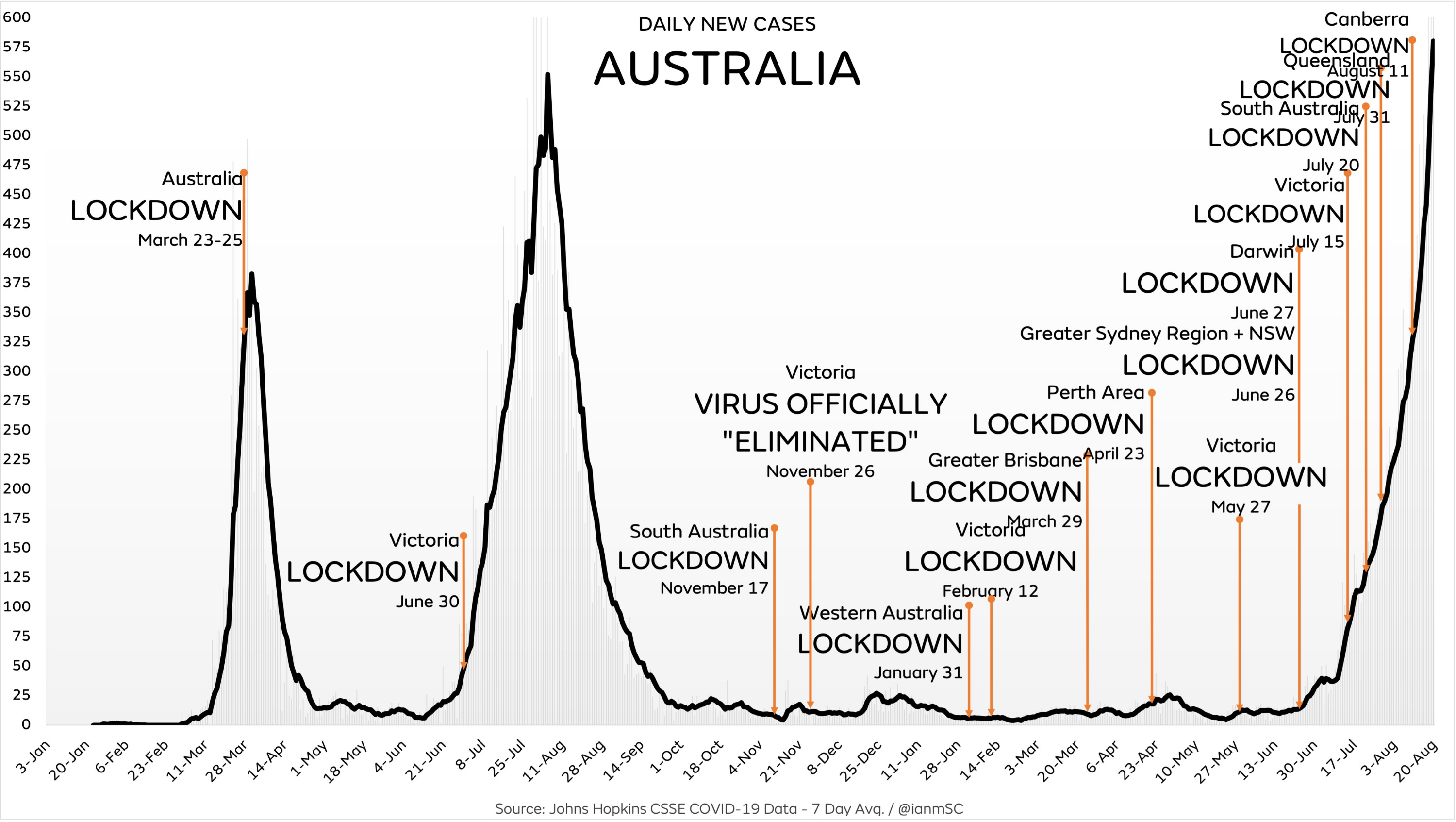
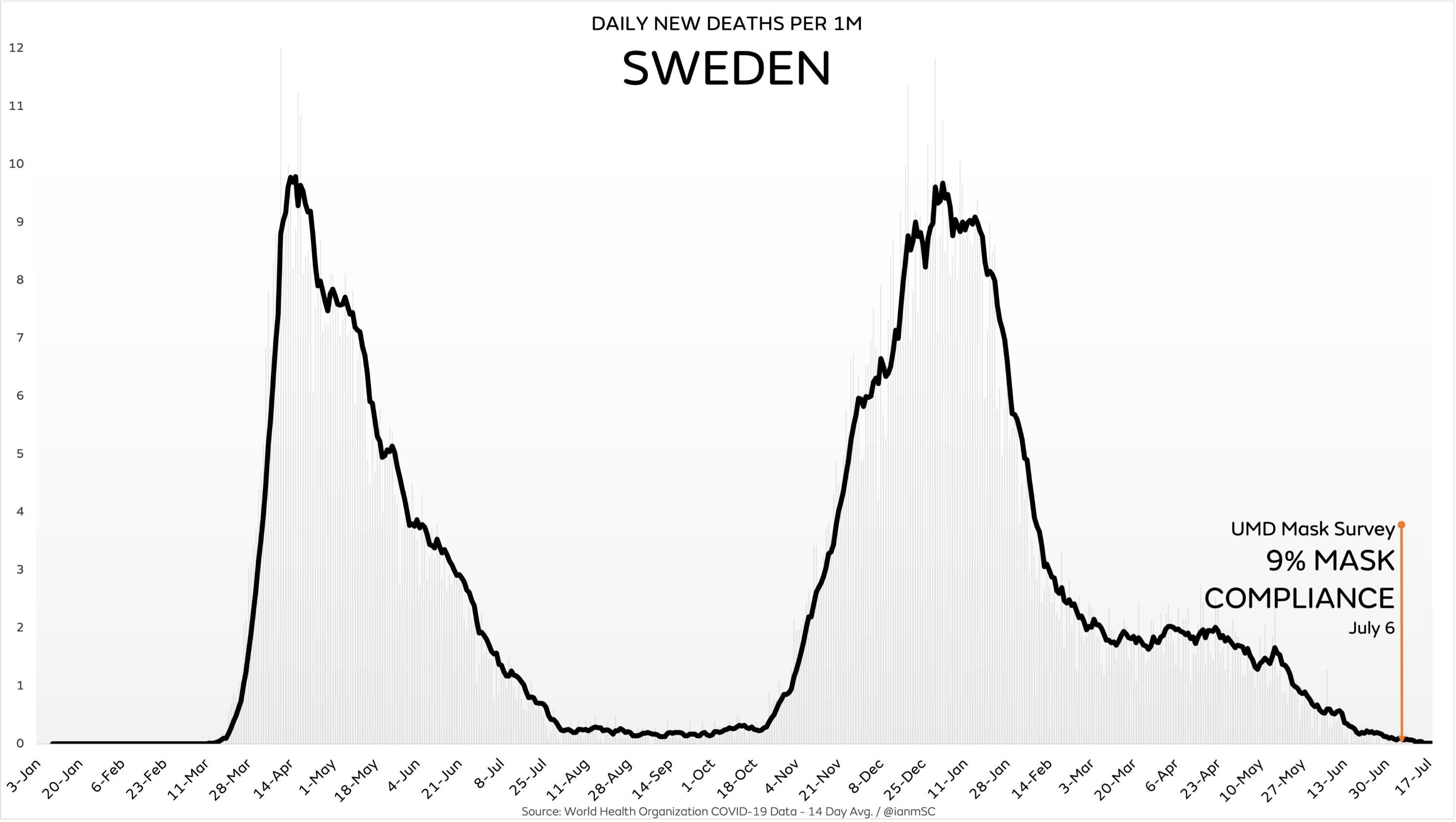
August 23, 2021
The FDA should demand adequate, controlled studies with long term follow up, and make data publicly available, before granting full approval to covid-19 vaccines, says Peter Doshi.
But here we are, with FDA reportedly on the verge of granting a marketing license 13 months into the still ongoing, two year pivotal trial, with no reported data past 13 March 2021, unclear efficacy after six months due to unblinding, evidence of waning protection irrespective of the Delta variant, and limited reporting of safety data. (The preprint reports “decreased appetite, lethargy, asthenia, malaise, night sweats, and hyperhidrosis were new adverse events attributable to BNT162b2 not previously identified in earlier reports,” but provides no data tables showing the frequency of these, or other, adverse events.)
Iraeli Ministry has Pfizer at 17% efficacy and is already reporting booster failures. Mayo Clinic has Pfizer at 42% protection among Rochester MN patients. Should be NO approval based on failed efficacy and clearly off the market for poor safety.
https://blogs.bmj.com/bmj/2021/08/23/does-the-fda-think-these-data-justify-the-first-full-approval-of-a-covid-19-vaccine/